Journal of the International Telemedicine Academy, Vol. 1, No. 2, pp. 8-17
The use of Distortion Product Otoacoustic Emissions in a Universal Neonatal Hearing Screening (UNHS)program
S. HATZOPOULOS1, J. PETRUCCELLI2, M. ROSSI1, A. MARTINI1
1University of Ferrara, Audiology Department, Ferrara, Italy
2Worcester Polytechnic Institute, Dept. of Mathematical Sciences, Worcester, USA
sdh@dns.unife.it
Abstract:
We have conducted a comparison of three DPOAE protocols, testing cubic 2F1 - F2 distortion products, in order to define the most feasible protocol for a universal hearing screening program. The protocols used asymmetrical stimulus intensities ( L1 > L2) with a frequency ratio of 1.22, in the following format: : (P1), L1= 60, L2= 50 dB SPL; (P2), L1= 65, L2= 55 dB SPL; and (P3), L1= 75, L2= 65 dB SPL. Linear TEOAE responses evoked by click stimuli of 75 dB p.e. SPL were used as controls of the normal cochlear function. Five 2F1 - F2 frequencies (ref. to F2) 1.5, 2.0, 3.0, 4.0, 5.0 kHz were tested with a ILO-92 macro subroutine. The project included randomly selected recordings from 1200 well-baby nursery (WBN) infants (age 48 hr) and 50 very low birth weight NICU infants. Statistical analyses comparing the signal to noise ratios (S/N), at the predefined F2 frequencies, indicated that the P1 and P2 DPOAE protocols perform similarly. Significant S/N differences were observed in the P3 to P2 and P3 to P1 data-set comparisons. DPOAE scoring criteria were estimated from the P3 data-set, using one-sided distribution-free tolerance boundaries. The scoring criteria for a "pass" were estimated as a minimum S/N of 6.0, 7.0 and 6.0 dB at 2.0, 3.0 and 4.0 kHz respectively. In terms of feasibility, the P3 protocol generated responses in 98% of the WBN and 76% of the NICU infants. All three DPOAE protocols demonstrated smaller time-recording requirements than the TEOAE standard. The false-positive ratio for the NICU infants was estimated as 8%.
Key words: OAEs, transient otoacoustic emissions, distortion product otoacoustic emissions, TEOAEs, DPOAEs, linear TEOAE protocol, well babies, NICU infants, universal hearing neonatal screening.
Introduction
The etiology of deafness arising during childhood age is various and often unknown. Nevertheless the prevalence of hearing impairment among the children is high (5/1000) (Brackett et al, 1993). Severe genetic or congenital hearing loss is represented in about 1-2/1000 of well-babies ( White et al, 1993) and in 4-5% (Mason and Herrmann, 1998; White et al, 1995) of newborns which exhibit one or more audiological risk factor (White et al, 1993; Parving, 1984;Joint Committee on Infant Hearing Screening, 1990). It has been shown that approximately 50% of children identified with sensorineural hearing loss (SNHL) do not exhibit any risk factors at birth (Mauk and Beherens, 1991; Vohr and Maxon, 1996). Within this context, a hearing screening focused only on the "at risk group" will detect no more than half of the deaf children. To increase the detection of hearing loss at the youngest possible age , it is necessary to implement an optimized universal hearing screening program, testing both well baby nursery (WBN) and neonatal intensive care unit (NICU) infants.
The leading technology for neonatal hearing screening is currently based on otoacoustic emission protocols. Transiently evoked otoacoustic emissions (TEOAEs) are the choice of the majority of European and US screening programs (Mencher et al, 2001; Welzl-Muller and Stephan, 2001: Psarommatis et al, 2001; Torrico Roman et al, 2001; Zehnder et al, 2000;White et al, 1993; Norton et, 2000;Gravel et al, 2000). The TEOAE protocols have a high penetrance in the hearing screening studies, despite the fact that the distortion product otoacoustic emissions (DPOAEs) show a good frequency specificity (Brown and Kemp, 1984; Martin et al, 1987) and a very good noise-immunity (Nelson and Kimberly, 1992; Gorga et al; 1997; Hatzopoulos et al, 2001), in the 2- 4 kHz frequency range where most screening programs evaluate the hearing function .
The main goal of this study was to test the hypothesis of whether a DPOAE screening protocol can be used efficiently, or as efficiently as a TEOAE protocol, in a Universal Neonatal Hearing Screening program (UNHS). To attain this objective we have: (1) used a large sample size of WBN infants; (2) a sample of NICU infants (very low birth weight cases); and (3) we have tested three different DPOAE protocols (using a preset number of five tested frequencies) in order to find the DPOAE protocol offering the best quality of responses (high signal to noise ratios) and the best test-feasibility. Due to time restrictions in the NICU environment, the DPOAE protocol-comparison and scoring-criteria estimation was performed only on the data from the WBN infants. The best DPOAE protocol was applied on the data from the NICU infants to attain a test-feasibility estimate.
Materials and Methods
The WBN screening program at Ferrara University (and in the region of Emilia-Romagna) uses a three-stage protocol. To test WBN infants, TEOAEs are used in stages 1 and 2 (re-tests are repeated within 14 days from birth). An Auditory Brainstem Response (ABR) diagnostic evaluation is used in stage 3 (within 3 months from birth). Neonates without reproducible TEOAEs (see scoring criteria in the protocols section) in either ear, after stage 2 are referred to stage 3. The objective of the program is to provide effective clinical assistance (intervention) to hearing impaired subjects within the first 6 months of life (Yoshinaga-Itano, 1995).
Different screening procedures are used for the Neonatal Intensive Care Unit infants. Although TEOAEs are routinely used in stages 1 and 2, the re-testing is conducted within a 3 day interval, for a maximum of 6 re-test sessions. For stage 3, as in the case of the WBN infants a diagnostic ABR evaluation is used.
Subjects
A group of 1200, randomly selected, neonates (mean age 40.5 ±1.8 weeks) was tested during the second day of life during natural sleep and after feeding, in a quiet room in the well-baby nursery . The neonates were considered normal if they had a gestational age > than 37 weeks, a birth weight appropriated for their gestational age (AGA) and an Apgar score between 7 to 10 (1rst and 5th min). The normal cochlear function of each subject was evaluated by TEOAE scoring criteria (mentioned in the section below) and then the DPOAE responses were acquired.
A group of 50, randomly selected, NICU infants characterized by very low birth weight (mean age 33 weeks ± 2.3, mean weight 1.200 ± 250 g) was tested also with TEOAEs in a silent room to verify the presence of emissions and the normality of the cochlear function. The evaluation of these responses was based on scoring criteria derived from well-babies.
Equipment and employed protocols
The OAE recordings were collected with a portable ILO292 equipment running the ILO software version 5.60. In previous publications (Hatzopoulos et al, 1999;Hatzopoulos et al, 2000a;Hatzopoulos et al, 2000b) we have presented evidence suggesting that a linear TEOAE protocol outperforms its nonlinear counterparts (QuickScreen included) in terms of signal to noise (S/N) ratios, correlation and noise level values. The linear TEOAE recordings were elicited using stimuli of 72- 75 dB p.e. SPL with an acceptable noise level set at 52.0 dB p.e. SPL. Each recording was the average of a minimum of 50 sweeps (the max allowable number was 80). The TEOAEs were post-windowed by a 3.5-12.5 ms windowing function and in order to suppress muscular and respiration artifacts, the low TEOAE frequencies were removed by setting the bandwidth of the ILO recording to 1.5-5.0 kHz. An ear was considered normal when the S/N ratios from the corresponding linear TEOAE response, at 2.0, 3.0 and 4.0 kHz, ( scoring criteria) were higher or equal to 7.0, 10.0, and 9.0 dB respectively (Hatzopoulos et al, 1999) . For a "pass" condition both ears should have satisfied the TEOAE scoring criteria.
The DPOAEs were elicited, using three asymmetrical protocols ( L1 > L2) which were named as follows: (P1), L1= 60, L2= 50 dB SPL; (P2), L1= 65, L2= 55 dB SPL; and (P3), L1= 75, L2= 65 dB SPL. The rationale for the choice of these protocols was the following: Regarding the issue of asymmetrical and not equal intensity protocols, data from animal studies have indicated that a 10-15 dB SPL difference in the DPOAE stimulus primaries generates the highest amplitude cubic DPOAE responses (Lonsbury-Martin et al, 1993; Whitehead et al, 1998). Although this advantage decreases above 65 dB SPL (Gaskill and Brown, 1990), we have used a high level asymmetrical protocol to be able to compare the S/N ratios of the three protocols at the same frequencies (symmetrical protocols are referenced to the geometric mean and not to F2). Regarding the issue of the selected stimulus intensities : A number of studies have shown that at lower DPOAE stimulus intensities it is easier to assess possible cochlear insults (Mills et al, 1993; Quinonez, 1999; Shera et Guinan, 1999). For that purpose we have implemented in the present study the P1 protocol (60-50 dB SPL). It should be noted that a protocol presenting lower stimulus intensities (i.e 50-40 dB SPL) might have been more efficient in detecting cochlear insults, but considering the noise levels in the WBN and the NICU environment such a protocol would have produced low test-efficiency results. The protocol P2 is considered the default option of many DPOAE instruments, although there is no evidence in the literature supporting such a choice. Finally, the high stimulus intensity protocol P3 was designed to overcome the ambient and subject noise problems in the neonatal hearing testing areas. Similar high stimulus protocols have been suggested or implemented in previous studies (Norton et al, 2000; Hatzopoulos et al, 2001).
The DPOAE protocols used a frequency-ratio of 1.22. Five frequencies of the cubic distortion product 2F1 - F2 were tested with an ILO macro routine at 1.5, 2.0, 3.0, 4.0, and 5.0 kHz (referenced to F2). The advantage of using the ILO macro was that the program could collect data at the F2 frequency with the worst S/N ratio when the S/N ratios at other tested frequencies were higher than 10 dB. At each frequency a minimum of 32 averages was collected, with an acceptable noise level set at -10 dB SPL. It should be noted that the tested frequency of 5 kHz is not a common audiometric frequency, but it was chosen over the frequency of 6.0 kHz, because the frequency response of the ILO probe above 5.0 kHz is not flat.
Statistical Analyses
The optimization of the best DPOAE protocol was conducted only with data from the WBN infants. The NICU environment does not favor the recording of long sequences of OAE responses and it was decided to optimize the DPOAE protocol with the WBN infants and then to apply it on the NICU population.
In terms of performance-optimization, we have considered as best the DPOAE protocol generating responses with the highest signal to noise ratio at 2.0, 3.0, 4.0 and 5.0 kHz. To evaluate which protocol generates the best S/N ratios Hotelling's T2 test was applied. This multivariate procedure compares all the DPOAE variables (creating a test-vector) between two given setups, i.e. between P1 and P2 or P2 and P3 . Additional details on this procedure can be found in a previous publication (Hatzopoulos et al, 1999). Due to time restrictions two DPOAE protocols and the standard linear TEOAE protocol were tested at a time . We have used 600 WBN infants to test the P1 and P2 and 600 infants to test the P3 and the P2 DPOAE protocols. To avoid any biasing errors the protocol order (which protocol was applied first) was randomized. For 46 subjects it was not possible to record TEOAE responses (14 agitated subjects) or responses from a second DPOAE protocol (32 subjects), therefore these cases were excluded from the study. The actual datasets used in the study refer to 581 cases for P1 and P2 comparisons and 573 cases for the P3-P1 protocol comparison.
The definition of the normative DPOAE scoring criteria was conducted by using a free distribution (Hatzopoulos et al, 1999; Hatzopoulos et al, 2000b). The reason for using a free distribution method is that the DPOAE variables (S/N ratios at 2.0, 3.0, 4,0 kHz) are not normally distributed. In this context, it should be noted that inferences about population means (as in the case of Hotelling's test) tend to be robust, if the sample size is moderate to large since the Central Limit Theorem guarantees that the distribution of means is closer to normal than the distribution of the original data. The scoring criteria were estimated from the best DPOAE protocol according the results of Hotteling's T2 test. The scoring criteria provide us with a minimum estimate of normal performance, which is the lower tolerance bound of the estimated tolerance interval (for every tested frequency). The statistical premises of this calculation can be expressed with the following statement: we are 99% confident that the calculated tolerance interval (at " x" tested DOPAE frequency ) contains at least 95% of the S/N ratio values of the entire WBN population. Prior to the estimation of the DPOAE scoring criteria the DPOAE responses were evaluated with an apriori criterion, according to which a response was considered "pass" when the S/N ratios at 2.0, 3.0 and 4.0 kHz were higher or equal to 3 dB and the DPOAE amplitude at each tested frequency was > 0 dB SPL.
For all analysis a mainframe SAS statistical package was used.
Results
Description of the Data
Typical DPOAE responses from WBN and NICU infants, evaluated as "pass" are shown in Figure 1. The descriptive statistics from the TEOAE and DPOAE data-sets are presented in Table 1 (WBN) and Table 2 (NICU) . As it was expected the S/N ratios at the lower frequencies of 1.0 and 1.5 kHz show the highest variability, which is probably caused by respiration and muscular artifacts.
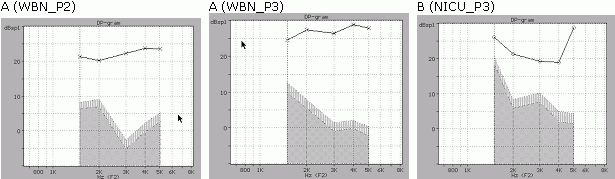
Figure 1. DP-gram data : (A) From a WMN infant (case 1322). The left panel shows the responses evoked by the P2 protocol (65-55 dB SPL) and the right panel the responses from the P3 protocol (75-65 dB SPL). In the latter there is a significant increase of the DPOAE amplitude level and a change in the shape of the noise level. By using a 75-65 protocol a DPOAE response can be clearly observed even in cases where the ambient noise is as high as 52 dB SPL. (B) a response from NICU infant (case P0054_007), elicited by the P3 protocol. The highest noise level was observed at the 1.5 kHz frequency, suggesting that the seal between the external meatus and the ILO neonatal probe was not optimized. In all three graphs the horizontal axis shows frequency in kHz and the vertical axis shows amplitude of the S/N ratio in dB.
Table 1. Descriptive statistics of the TEOAE and DPOAE variables from the 1200 WBN infants. The shaded cells indicate the beginning of a new protocol. The data variables presented are organized in the following order: TEOAE, DPOAE-P1, DPOAE-P2 and DPOAE-P3 protocols. The cases included in these 4 data-sets were evaluated as normal (normal cochlear function) by independent TEOAE and DPOAE criteria.
| OAE Variables | Mean | Standard Deviation |
|---|---|---|
| TEOAE S/N 1.0 kHz (dB) | 4.4 | 7.6 |
| TEOAE S/N 2.0 kHz (dB) | 16.9 | 4.9 |
| TEOAE S/N 3.0 kHz (dB) | 19.1 | 5.4 |
| TEOAE S/N 4.0 kHz (dB) | 19.1 | 5.9 |
| TEOAE S/N 5.0 kHz (dB) | 10.1 | 7.2 |
| TEOAE Response (dB SPL) | 21.6 | 5.1 |
| TEOAE Noise (dB SPL) | 3.9 | 2.2 |
| TEOAE Time (sec) | 54.3 | 35.3 |
| DPOAE (P1) S/N 1.5 kHz (dB) | 11.5 | 5.7 |
| DPOAE (P1) S/N 2.0 kHz (dB) | 14.8 | 5.8 |
| DPOAE (P1) S/N 3.0. kHz (dB) | 14.1 | 6.2 |
| DPOAE (P1) S/N 4.0 kHz (dB) | 17.5 | 7.3 |
| DPOAE (P1) S/N 5.0 kHz (dB) | 21.2 | 7.6 |
| DPOAE (P1) Time (sec) | 36.3 | 17.4 |
| DPOAE (P2) S/N 1.5 kHz (dB) | 11.6 | 6.4 |
| DPOAE (P2) S/N 2.0 kHz (dB) | 15.1 | 6.4 |
| DPOAE (P2) S/N 3.0. kHz (dB) | 14.9 | 6.4 |
| DPOAE (P2) S/N 4.0 kHz (dB) | 16.4 | 7.2 |
| DPOAE (P2) S/N 5.0 kHz (dB) | 21.2 | 7.8 |
| DPOAE (P2) Time (sec) | 35.3 | 15.9 |
| DPOAE (P3) S/N 1.5 kHz (dB) | 11.9 | 7.9 |
| DPOAE (P3) S/N 2.0 kHz (dB) | 17.9 | 8.1 |
| DPOAE (P3) S/N 3.0. kHz (dB) | 20.3 | 7.4 |
| DPOAE (P3) S/N 4.0 kHz (dB) | 20.8 | 8.4 |
| DPOAE (P3) S/N 5.0 kHz (dB) | 25.7 | 9.5 |
| DPOAE (P3) Time (sec) | 29.1 | 11.2 |
Table 2. Descriptive statistics of the TEOAE and DPOAE variables from the 50 NICU infants. The shaded cells indicate the beginning of a new protocol.
| OAE Variables | Mean | Standard Deviation |
|---|---|---|
| TEOAE S/N 1.0 kHz (dB) | 3.7 | 8.1 |
| TEOAE S/N 2.0 kHz (dB) | 9.8 | 6.9 |
| TEOAE S/N 3.0 kHz (dB) | 13.2 | 7.7 |
| TEOAE S/N 4.0 kHz (dB) | 13.5 | 7.5 |
| TEOAE S/N 5.0 kHz (dB) | 10.1 | 7.2 |
| TEOAE Response (dB SPL) | 16.9 | 8.1 |
| TEOAE Noise (dB SPL) | 5.5 | 3.6 |
| DPOAE (P3) S/N 1.5 kHz (dB) | 11.9 | 7.9 |
| DPOAE (P3) S/N 2.0 kHz (dB) | 12.8 | 8.0 |
| DPOAE (P3) S/N 3.0. kHz (dB) | 13.3 | 9.0 |
| DPOAE (P3) S/N 4.0 kHz (dB) | 14.6 | 8.3 |
| DPOAE (P3) S/N 5.0 kHz (dB) | 15.7 | 9.4 |
The distribution of the S/N ratios of DPOAE responses from the P1 and P2 protocols presented skewed patterns suggesting that the DPOAE variables were not normally distributed. The skewness was less pronounced in the P3 DPOAE data-set. Normalized quantile plots of various S/N ratios from the P1, P2 datasets presented evidence of bimodal distributions. Figure 2 shows normalized plots of the S/N ratio at 3.0 kHz from the P1, P2 and P3 datasets. The little "bump" in the graphs 2_P1 and 2_P2 of Figure 2 is an indication of a bimodal behavior.
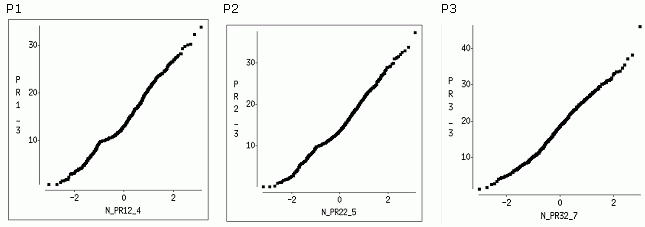
Figure 2. Normal quantile plots of the S/N DPOAE responses at 3.0 kHz elicited by P1, P2 and P3 DPOAE protocols. In the first two graphs a "bump" is shown, suggesting that the data have a bimodal distribution. The horizontal axis shows the normalized quantile values, while the vertical axis shows amplitude of the S/N ratio in dB.
Comparison of DPOAE protocols
The results from the protocol comparisons are shown in Table 3. The comparison between protocols P1 and P2 indicated that there were no significant S/N ratio differences at the frequencies 1.5 , 2.0 and 5.0 kHz. Significant differences were observed at the frequencies 3.0 and 4.0 kHz. The P1 protocol produced larger responses at 3.0 kHz while the P2 protocol produced larger responses at 4.0 kHz. Considering that the responses from these two tested protocols were quite similar, only an additional comparison was conducted (P3 vs. P2). For the latter, the data indicated that for all tested frequencies the S/N ratio differences were significant . Since the DPOAE responses from the P3 protocol presented the largest S/N ratios, the P3 protocol was considered as the one generating the best quality of DPOAE responses and the best candidate for a UNHS program.
Table 3. Results from the DPOAE protocol comparison using Hotelling's T2 test . The second column shows the differences between protocols P1 and P2 and the third column shows differences between protocols P3 and P2. Since the responses from P1 were found very similar to the responses of P2 no comparison between P3 and P1 was conducted.
| DPOAE Variables | P1 vs. P2 CI= 99% | P3 Vs P2 CI= 99% |
|---|---|---|
| S/N 1.5 kHz | Ns | * |
| S/N 2.0 kHz | Ns | * |
| S/N 3.0 kHz | * (better P1) | * |
| S/N 4.0 kHz | * (better P2) | * |
| S/N 5.0 kHz | Ns | * |
|
P1 = 60-50 dB SPL, P2= 65-55 dB SPL, P3= 75-65 dB SPL * = significant differences ns = no significant differences CI= Confidence Interval |
||
Estimation of scoring criteria
With the strict application of the apriori 3 dB criterion on the DPOAE responses of the P3 data-set, the number of available cases was reduced to 560 (573-13). The thirteen cases which were excluded (in order to avoid any outliers in the data distribution) presented S/N ratios well above 3 dB, but DPOAE amplitude levels between -0.5 and -3.0 dB SPL at the frequencies 3.0 or 4.0 kHz.
The results from the free distribution confidence interval estimation are shown in Table 4. According to these, a DPOAE response is considered a "pass" when both the left and right ear responses have S/N ratios at 2.0, 3.0 and 4.0 kHz higher than 6.0, 7.0 and 6.0 dB respectively. For this set of criteria there is no need to include additional rules regarding the DPOAE amplitude level at each tested frequency. In all 560 cases the DPOAE amplitudes at 2.0, 3.0 , 4.0 and 5.0 kHz were larger than 0 dB SPL. For comparison purposes Table 4 includes values of scoring criteria, computed via a free distribution (Hatzopoulos et al, 1999), from normal neonatal TEOAE recording elicited by a linear TEOAE protocol.
Table 4. Scoring criteria for DPOAE and TEOAE protocols for 2.0, 3.0 and 4.0 kHz. The table presents also data from a non-audiometric frequency at 5.0 kHz (shaded cells) , which might offer useful information about basal cochlear functionality. The TEOAE scoring criteria have larger values because the TEOAE click stimulus (on the average of 76 dB SPL) is larger than the intensity of the L2 tone (mainly responsible for the generation of the cubic distortion product in an asymmetrical DPOAE protocol).
|
Frequencies (kHz) |
P3 - DPOAE protocol (dB) |
Linear TEOAE protocol (dB) |
|---|---|---|
| 2.0 | 6.0 | 8.0 |
| 3.0 | 7.0 | 11.0 |
| 4.0 | 6.0 | 10.0 |
| 5.0 | 8.0 | 10.0 |
Feasibility of the DPOAE testing
The screening yields (feasibility index) of the 75-65 DPOAE protocol were estimated as follows: For the available WBN infants we have considered that the test was feasible in 560 cases ( 560 / 573 = 98%) which produced DPOAE responses with acceptable S/N ratios. The calculation of the feasibility estimate from the DPOAE responses of the NICU residents was more complicated. The reason for this complexity was that a number of NICU neonates did not produce a DPOAE response even when the TEOAE response was considered a "pass", and that in 6 cases no TEOAE response was present at the initial test. The main contributor to the lack of DPOAE responses was the size of the neonatal DPOAE probe, whose diameter was probably large for the meatus size of the preterm infants (note that the neonatal TEOAE probe is considerably thinner). To overcome this technical difficulty we established a rule by which we considered a DPOAE response as valid within 4 re-tests for which TEOAEs were present sequentially at least in 2 out of the 4 re-tests. After the application of this rule 38 infants (38 / 50 = 76%) were evaluated as "pass" cases according to the established DPOAE criteria.
For the well baby population no ABR testing is conducted once the case is considered a "pass". In contrary due to the small number of NICU residents (approximately 12% of the total number of births in our hospital, which translates to approx. 140 cases per year) it is possible to perform a diagnostic ABR independently of the outcome of the OAE testing. For the 38 cases tested with DPOAEs, a diagnostic ABR at 50 dB nHL indicated 3 cases with monolateral losses ( i.e. "fail cases). The corrected age of these three infants at the time of the ABR test was greater than 40 weeks. The results from the first ABR were considered as non-conclusive, due to issues related to a possible non-maturation of the central pathways. A second ABR test after 8 weeks indicated that 2 of the 3 cases had normal ABR latency values. According to these data the false negative estimate of the DPOAE testing in the NICU was equal to 1/ 38 = 2.6%. From the 12 infants which did not satisfy the scoring criteria, ABR testing identified 3 bilateral and 5 monolateral hearing impairment cases. In this context, the false positive estimate of the DPOAEs was equal to 4 / 50 = 8%. For comparison purposes we present the data from the TEOAE recordings. From the 12 cases , where a DPOAE evaluation was not possible, 5 resulted as fail and 7 as partial pass since all these cases the S/N limit at 2.0 kHz was not satisfied. The ABR evaluation (at 50 dB nHL) has indicated that 4 of the partial pass cases presented monolateral losses. In this context ,it might be said that the false-negative ratio of the TEOAEs was 7 / 50 = 14%.
Discussion
The objective of this study was the evaluation of the clinical performance of a DPOAE screening protocol , in the context of UNHS program. The presented data suggest that a DPOAE cochlear evaluation, at 3 pre-selected frequencies, has a good test feasibility (98%) for the WBN infants and an average feasibility (76%) for the NICU infants.
From the three proposed DPOAE protocols, the high stimulus intensity asymmetrical protocol (75-65) presented responses with the highest S/N ratios. The advantage of using high level primaries is that a cochlear response can be elicited even in a noisy environment. It might be argued that the high level primaries might elicit responses even from subjects with mild mid-frequency hearing losses, but it should be noted that the current TEOAE programs are using click stimuli (optimized in the mid-frequencies) as high as 85 dB SPL, 10 dB higher than the L1 of the suggested protocol.
In terms of recording time requirements, significant differences were found between the tested WBN DPOAE and TEOAE (mean 54 ± 32 s) time estimates with the latter showing larger mean values. No significant differences were found between the recording times from the P1 (mean 36 ±17.2 s) and P2 (mean 35 ±15.9 s) protocols. As expected significant differences were found between the recording times of P3 (mean 29 ± 11.2 s) and P2 , P1. These recording time differences can be attributed to the fact that the DPOAE protocols stimulate in a more efficient manner the cochlea, therefore more robust DPOAE responses contribute to fewer necessary recording averages. For the NICU infants the recording time requirements are not an important issue, because several TEOAE or DPOAE re-tests are necessary in order to obtain an acceptable OAE response.
The characteristics of the DPOAE recordings of our study resemble the data reported by previous studies for WBN infants (Abdala, 1996; 2000;Quinonez, 1999; Norton et al, 2000; Gordts et al, 2000) and NICU infants (Smurzinsky, 1993; Gorga et al, 2000). In our study the majority of the WBN DPOAE responses had profiles similar to Figure 1. Approximately 10% of the responses of the P1 and P2 datasets and 5% of the responses of the P3 data-set, presented what we might call a DP-gram notch pattern. Similar observations have been presented in a previous study by Marco et al, where the DPOAEs were described as presenting peaks at 2.0 and 5.0 kHz and "valleys or notches" in between. Representative responses of the notch in the DP-gram are shown in Figure 3. We have postulated that this "notch" behavior around the DPOAE frequency of 3 kHz (referenced to F2) might have been caused by a number of factors: (a) by an interaction between the DPOAE response (referenced to F2) and a nearby peak of a spontaneous emission; (b) by an interaction between the DP cubic response and a standing wave in the external meatus; and (c) by a particular resonance of the ILO-92 probe. The fact that the "DP-gram notch" was observed in only a small percentage of the tested cases favors more the first two postulates. The hypothesis of an interaction between cubic DPOAE responses and a spontaneous emission is quite possible, but one should expect interactions in other frequencies as well (i.e. at 2.0 or 4.0 kHz). Within this context we consider more probable the standing wave hypothesis, which depends on the position of the ILO-probe and the dimensions of the external auditory meatus (note: The DP-gram notch usually becomes less profound when the probe is positioned closer to the tympanic membrane). Additional support for the first hypothesis was derived from the NICU preterm data-set. Approximately in 29% (11 cases) of infants tested with the P3 protocol, a notch pattern was visible in the DP-gram. In the preterm cases the DPOAE probe was positioned further away from the entrance of the auditory meatus, due to large diameter of the probe. Despite this confirmation, additional analyses are considered necessary in order to resolve the origin of this particular DP-gram behavior.
Regarding the bimodal structure of the data, we postulate that several factors might be responsible, such as : (a) a change of the number of active components, in the DPOAE response, to passive components which are less sensitive to the incoming vibrations. Such an change is taking place approximately around the stimulus level of 65 dB SPL (Mills et al, 1993; Whitehead et al, 1995 ) ; and (b) parameters related to the recording instrument (ILO-92). The data from this study do not offer a satisfactory explanation, therefore further studies are necessary to elucidate the issue of the bimodal structure of the DPOAE data.
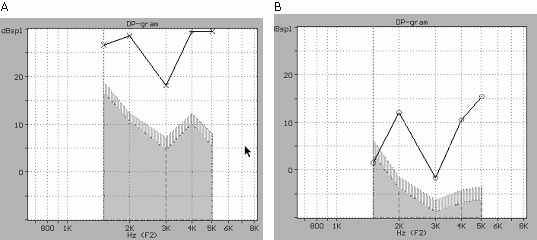
Figure 3. DP-gram from a 75-65 protocol for : (A) a WBN infant (case 2246_L001) and (B) from a NICU infant (case P0031_007). The DP-gram "notch" at 3.0 kHz is considered as a technical artifact, caused by the interaction of the DPOAE response and a standing wave, and not an indication of a cochlear impairment. The horizontal axis shows frequency in kHz and the vertical axis the amplitude of the S/N ratio in dB. The "depth" of the notch is minimized by a repositioning of the DPOAE probe.
In terms of screening efficiency in the WBN environment, both TEOAE and DPOAE (P3) protocols perform equally well. This high DPOAE yield permits us to postulate that the DPOAEs might outperform the linear TEOAEs in noisy WBN environments, due to the fact that the energy delivery to the cochlea of the DPOAE protocol is more efficient. The S/N ratios at 5 kHz showed the highest values among the tested frequencies, thus it is conceivable that by using a DPOAE screening test with 4 frequencies, we might evaluate more accurately the basal cochlear partitions.
The screening efficiency of DPOAEs in the NICU infants was average and only 76% of the cases presented acceptable responses. The reported false-positive estimate was 8%, a value lower than the data reported by Barker et al, referring to DPOAE false-positive rates of 11% to 35%. Although these estimates are bound to change with the use of larger data-sets, it should be mentioned that some bias is introduced to the results by employing screening practices which are not fully optimized. For example we have identified that in the NICU screening a major component of the "fail" cases is related to technical problems ( i.e. the DPOAE probe is too large in comparison to the auditory meatus). Analyses of variance of the NICU DPOAE responses have indicated that the majority of the "fail" responses were corresponding to an age between 30 and 32 weeks. Testing at a latte age, for example at 35th week, might provide better feasibility scores and lower false-positive rates.
In conclusion, the data of the study suggest that a high stimulus DPOAE protocol can be used in a UNHS program. Although the DPOAEs have significant lower time-recording requirements the data show that both TEOAEs and DPOAEs perform equally well on the WBN infants. This implies that hybrid programs can be designed using DPOAEs in the noisiest testing site (i.e stage 1 or stage 2) . For the NICU infants the data indicate that some technical problems must be resolved first ( i.e. smaller probe sizes), before a proper evaluation of the OAE protocols is conducted. The data of this study suggest that a DPOAE protocol cannot evaluate properly all the NICU infants and it is strongly suggested to employ a clinical program combining multiple sessions of DPOAEs /TEOAEs and ABR.
Acknowledgements
The authors would like to thank Mrs. Camurri for technical assistance with the ABR recordings. Funding for this research was provided by a grant of the Emilia-Romagna Region.
References
- Abdala C: Distortion product otoacoustic emission (2f1-f2) amplitude as a function of f2/f1 frequency ratio and primary tone level separation in human adults and neonates. J Acoust Am 1996;100:3726-3740.
- Abdala C: Distortion product otoacoustic emission (2f1-f2) amplitude growth in human adults and neonates. J Acoust Am 2000;107:446-456.
- Barker SE, Lesperance MM, Kileny PR: Outcome of newborn hearing screening by ABR compared with different DPOAE pass criteria. Am J Audiol 2000;9:142-148.
- Brackett D, Maxon AB, Blackwell PM: Intervention issues created by successful universal newborn hearing screening. Semin Hear 1993;14:88-104.
- Brown AM, Kemp DT: Suppressability of the 2F1-F2 stimulated otoacoustic emission in gerbil and man. Hear Res 1984;13:29-37.
- Clems CJ, Davis SA: Minimizing false-positives in universal newborn hearing screening. A simple solution. Pediatrics 2001, 107:E29
- Das VK: Aetiology of bilateral sensorineural hearing impairment in children: a 10 year study. Arch Disease Childhood 1996;74:8-12.
- Gaskill SA, Brown AM: The behavior of the acoustic distortion product, 2f1-f2, from the human ear and its relation to auditory sensitivity. J Acoust Soc Am 1990;88:821-839.
- Gorga MP, Neely ST, Ohlrich B, Hoover B, Redner J, Peters J: From laboratory to clinic: A large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear 1997;18:440-455.
- Gorga MP, Norton SJ, Sininger YS, Cone-Wesson B, Folsom RC, Vohr BR, Widen JE, Neely ST: Identification of neonatal hearing impairment: distortion product otoacoustic emissions during the perinatal period. Ear Hear 2000;21:400-424.
- Gordts F, Naessens B, Mudde CA, Clement PA: Reference data for DPOAE in healthy newborns. Scand Audiol 2000;29:79-82
- Gravel J, Berg A, Bradly M, Cacace A, Campell D, Dalzell L, DeCristoforo M, Greenberg E, Gross S, Orlado M, Pinheiro J, Regan J, Spivak L, Stevens B: New York universal newborn hearing screening demonstration project: effects of screening protocol on inpatient outcome measures. Ear Hear 2000;21:131-140.
- Hatzopoulos S, Cheng J, Grzanka A, Martini A: On the optimization of the TEOAE recording protocols. A linear protocol derived from parameters of a time-frequency analysis. Data from neonatal subjects. Scand Audiol. 2000;29:21-7.
- Hatzopoulos S, Petruccelli J, Pelosi G, Martini A: A TEOAE screening protocol based on linear click stimuli: performance and scoring criteria. Acta Otolaryngol. 1999 ;119:135-9.
- Hatzopoulos S, Tsakanikos M, Grzanka A, Ratynska J, Martini A: A comparison of neonatal TEOAE responses recorded with linear and QuickScreen protocols. Audiology 2000;39:70-79.
- Hatzopoulos S, Pelosi G, Petruccelli J, Rossi M, Vigi V, Chierici R, Martini A: Efficient otoacoustic emission protocols employed in a hospital-based neonatal screening program. Acta Otolaryngol 2001;121:269-273.
- Joint Committee on Infant Hearing 1990 position statement. ASHA Suppl. 1991;33:3-6.
- Lonsbury-Martin BL, McCoy MJ, Whitehead ML, Martin GK: Clinical testing of Distortion-Product Otoacoustic Emissions. Ear Hear 1993;1:11-22.
- Marco J, Morant A, Caballero J, Ortells I, Paredes C, Brines J: Distortion-product otoacoustic emissions in healthy newborns: normative data. Acta Otolaryngol (Stockh) 1995;115:187-9.
- Martin G, Probst R, Coats AC, Lonsbury-Martin BL: Acoustic distortion products in rabbits, II: sites of origin revealed by suppression and pure-tone exposures. Hear Res 1987;28:191-208.
- Mason JA, Herrmann KR: Universal infant hearing screening by automated auditory brainstem response measurement. Pediatr 1998;10:42-9.
- Mauk GW, White KR, Mortensen LB, Beherens TR: The effectiveness of hearing programs based on high-risk characteristic in early intervention of hearing impairment. Ear Hear 1991;12:312-9.
- Mencher GT, Davis AC, DeVoe SJ, Beresford D, Bamford JM: Universal neonatal screening :past, present and future. Am J Audiol 2001;10:3-12.
- Mills DM, Norton SJ, Rubel EW: Vulnerability and adaptation of distortion product otoacoustic emissions to endocochlear potential variation. J Acoust Soc Am 1993;94:2108-2122.
- Nelson DA, Kimberley BP: Distortion-product emissions and auditory sensitivity in human ear with normal hearing and cochlear hearing loss. J Speech Hear Res 1992;35:1142-59.
- Norton SJ, Gorga MP, Widen JE, Folsom RC, Sininger Y,
Cone-Wesson B, Vohr BR, Mascher, K,
Fletcher K: Identification of neonatal hearing impairment: evaluation of transient evoked otoacoustic emission, distortion product otoacoustic emission, and auditory brain stem response test performance.
Ear Hear 2000 ;21:508-528. - Parving A: Aetiological diagnosis in hearing-impaired children-, clinical value and application of a modern examination programme. Int J Pediatr Otorhinol 1984;7:29-38.
- Psarommatis IM, Tsakanikos MD, Diamantopoulou PM, Douniadakis DE, Apostolopoulos NK: Towards a universal newborn hearing screening. Scand Audiol Suppl 2001;52:25-27.
- Quinonez RE: Distortion-product otoacoustic emissions (DPEs) in neonates: frequency ratio (F2/F1) and stimulus level differences (L1-L2). Acta Otolaryngol (Stoch) 1999;119:431-436.
- Ruben R, Rapin I: Plasticity of the developing auditory system. Ann Otol Rhinol Laryngol 1980;89:303-11.
- Shera CA, Guinan JJ Jr: Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs. J Acoust Soc Am 1999;105:782-795.
- Smurzynski J: Longitudinal measurements of distortion-product and click-evoked otoacoustic emissions of preterm infant: preliminary results. Ear Hear 1994;15:210-23.
- Torrico Roman P, Trinidad Ramos G, de Caceres Morillo MC, Lozano S, Lopez-Rios Velasco. An Esp Pedatr 2001;54:283-289.
- Vohr BR, Maxon AB: Screening infants for hearing impairment. J Pediatr 1996;128:710-4.
- Welzl-Muller K, Stephan K: Examples of implemented neonatal hearing screening programs in Austria. Scand Audiol Suppl 2001;52:7-9.
- White KR, Culpepper B, Maxon AB, Vohr BR, Mauk GW: Transient evoked otoacoustic emission-based screening in typical nurseries: a response to Jacobson and Jacobson. Int J Pediatr Otorhinol 1995;33:17-21.
- White KR, Vohr BR, Behrens TR: Universal newborn hearing screening using transient evoked otoacoustic emissions: results of the Rhode Island Hearing Assessment Project. Semin Hear 1993;14:18-29.
- Whitehead ML, McCoy MJ, Lonsbury-Martin BL, Martin GK: Dependence of distortion-product otoacoustic emissions on primary levels in normal and impaired ears. I. Effects of decreasing L2 below L1. J Acoust Soc Am 1995;97:2346-2358.
- Yoshinaga-Itano C: Efficacy of early identification and early intervention. Semin Hear 1995;16:115-123.
- Zender A, Probst R, Vischer M, Linder T: First results of a national hearing screening program in Switzerland. Schweiz Med Wochenchr 2000;71S-74S.
